Hi All,
Congratulations! Once again Loon Lake water appears to be in good shape. See attachment for 2008 report.
You can help keep it that way by following the recommendations
put forth by the Adirondack Watershed Institute at Paul Smith's College for insuring the continuation of good water quality as follows:NOTE:
The above captioned report refers only to water quality in terms of clarity, pH, conductivity, etc. The testing it reflects did not address the presence of invasive species. We will be conducting an aquatic plant survey during late summer of this year to determine the presence of any invasive plants or zebra muscles.Best Wishes to you all,
Vince
Introduction
The Adirondack Lake Assessment Program is a volunteer monitoring program established by the Residents’ Committee to Protect the Adirondacks (RCPA) and the Adirondack Watershed Institute (AWI). The program is now in its’ eleventh year and continues to grow. The program was established to help develop a current database of water quality in Adirondack lakes and ponds. There were 75 participating lakes in the program in 2008.
Methodology
Each month participants (trained by AWI staff) measured transparency with a secchi disk and collected a 2-meter composite of lake water for chlorophyll-a analysis and a separate 2-meter composite for total phosphorus and other chemical analyses. The participants filtered the chlorophyll-a sample prior to storage. Both the chlorophyll-a filter and water chemistry samples were frozen for transport to the laboratory at Paul Smith’s College.
In addition to the volunteer samples, AWI staff sampled water quality parameters in most of the participating lakes as time and weather allowed. In most instances, a 2-meter composite of lake water was collected for chlorophyll-a analysis. Samples were also collected at depths of 1.5 meters from the surface (epilimnion) and within 1.5 meters of the bottom (hypolimnion) for chemical analysis. Once collected, samples were stored in a cooler and transported to the laboratory at Paul Smith’s College.
All samples were analyzed AWI staff in the Paul Smith’s College laboratory using the methods detailed in Standard Methods for the Examination of Water and Wastewater, 21st edition (Greenberg, et al, 2005). Volunteer samples were analyzed for pH, alkalinity, conductivity, color, nitrate, chlorophyll a and total phosphorus concentrations. Samples taken by AWI staff were analyzed for the same parameters, as well as for calcium, chloride, and aluminum concentrations.
Results Summary
Loon Lake was sampled four times by volunteers, once with AWI staff, in the North Basin in 2008. Samples were collected on the following dates: 6/08/08, 7/06/08, 8/05/08, and 8/31/08. Loon Lake was sampled three times by volunteers in the South Basin in 2008. Samples were collected on the following dates: 6/08/08, 7/06/08, and 8/31/08. Results for 2008 are presented in Appendix A and will be discussed in the following sections. Results are presented as concentrations in milligrams per liter (mg/L) or its equivalent of parts per million (ppm) and micrograms per liter (mg/L) or its equivalent of parts per billion (ppb).
1 mg/L = 1 ppm; 1 mg/L = 1 ppb; 1 ppm = 1000 ppb.
Adirondack lakes are subject to the effects of acidic precipitation (i.e., snow, rain). A waterbody’s susceptibility to acid producing ions is assessed by measuring pH, alkalinity, calcium concentrations, and the Calcite Saturation Index (CSI). These parameters define both the acidity of the water and its buffering capacity. Based on the results of the 2008 Adirondack Lake Assessment program, Loon Lake’s acidity status is considered to be satisfactory, with a low sensitivity to further acidic inputs.
Limnologists, the scientists who study bodies of fresh water, classify lake health (trophic status) into three main categories: oligotrophic, mesotrophic, and eutrophic. The trophic status of a lake is determined by measuring the level of three basic water quality parameters: total phosphorus, chlorophyll-a, and secchi disk transparency. These parameters will be defined in the sections that follow. Oligotrophic lakes are characterized as having low levels of total phosphorus, and, as a consequence, low levels of chlorophyll-a and high transparencies. Eutrophic lakes have high levels of total phosphorus and chlorophyll-a, and, as a consequence, low transparencies. Mesotrophic lakes have moderate levels of all three of these water quality parameters. Based upon the results of the 2008 Adirondack Lake Assessment Program, Loon Lake is considered to be late oligotrophic.
PH
The pH level is a measure of acidity (concentration of hydrogen ions in water), reported in standard units on a logarithmic scale that ranges from 1 to 14. On the pH scale, 7 is neutral, lower values are more acidic, and higher numbers are more basic. In general, pH values between 6.0 and 8.0 are considered optimal for the maintenance of a healthy lake ecosystem. Many species of fish and amphibians have difficulty with growth and reproduction when pH levels fall below 5.5 standard units. Lake acidification status can be assessed from pH as follows:
pH less than 5.0 Critical or Impaired
pH between 5.0 and 6.0 Endangered or Threatened
pH greater than 6.0 Satisfactory or Acceptable
The pH in the upper water of Loon Lake ranged from 6.81 to 7.12. The average pH was 6.90 in the North Basin. The average pH in the South basin was the same at 6.90. Based solely on pH, Loon Lake’s acidity levels should be considered satisfactory.
Alkalinity
Alkalinity (acid neutralizing capacity) is a measure of the buffering capacity of water, and in lake ecosystems refers to the ability of a lake to absorb or withstand acidic inputs. In the northeast, most lakes have low alkalinities, which mean they are sensitive to the effects of
acidic precipitation. This is a particular concern during the spring when large amounts of low pH snowmelt runs into lakes with little to no contact with the soil’s natural buffering agents. Alkalinity is reported in milligrams per liter (mg/L) or microequivelents per liter (meq/L). Typical summer concentrations of alkalinity in northeastern lakes are around 10 mg/l (200 meq/L).
Lake acidification status can be assessed from alkalinity as follows:
Alkalinity less than 0 ppm Acidified
Alkalinity between 0 and 2 ppm Extremely sensitive
Alkalinity between 2 and 10 ppm Moderately sensitive
Alkalinity between 10 and 25 ppm Low sensitivity
Alkalinity greater than 25 ppm Not sensitive
The alkalinity of the upper water of Loon Lake ranged from 18.4 ppm to 28.6 ppm. The average alkalinity was 22.7 ppm in the North Basin. The average alkalinity for the South Basin was a similar 23.7 ppm. These values indicate that Loon Lake has a low sensitivity to acidification.
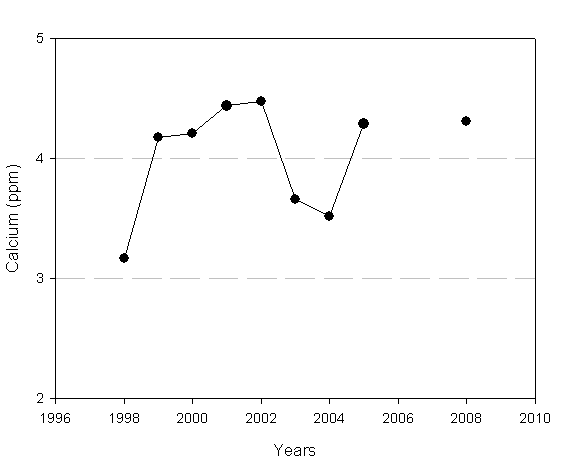
Calcium
Calcium is one of the buffering materials that occur naturally in the environment. However, it is often in short supply in Adirondack lakes and ponds, making these bodies of water susceptible to acidification by acid precipitation. Calcium concentrations provide information on the buffering capacity of that lake, and can assist in determining the timing and dosage for acid mitigation (liming) activities. Adirondack lakes containing less than 2.5 ppm of calcium are considered to be sensitive to acidification.
The calcium in the upper water of Loon Lake was 4.31 ppm in 2008 in the North Basin. In the bottom water, the calcium concentration was 4.46 ppm in 2008. This suggests that Loon Lake may currently not be sensitive to acidification.
Calcite Saturation Index
The Calcite Saturation Index (CSI) is another method that is used to determine the sensitivity of a lake to acidification. High CSI values are indicative of increasing sensitivity to acidic inputs. CSI is calculated using the following formula:
Ca Alk
CSI = - log10 40000 - log10 50000 – pH + 2
Where Ca = Calcium level of water sample in ppm or mg/L
Alk = Alkalinity of the water sample in ppm or mg/L
pH = pH of the water sample in standard units
Lake sensitivity to acidic inputs is assessed from CSI as follows:
CSI greater than 4 Very vulnerable to acidic inputs
CSI between 3 & 4 Moderately vulnerable to acidic inputs
CSI less than 3 Low vulnerability to acidic inputs
CSI values for Loon Lake were found to be 2.60 in the sample taken from the upper water in the North Basin, and 2.52 in the bottom water sample in 2008. These values classify Loon Lake as having low vulnerability to further acidic inputs.
Total Phosphorus
Phosphorus is one of the three essential nutrients for life, and in northeastern lakes, it is often the controlling, or limiting, nutrient in lake productivity. Total phosphorus is a measure of all forms of phosphorus, both organic and inorganic. Total phosphorus concentrations are directly related to the trophic status (water quality conditions) of a lake. Excessive amounts of phosphorus can lead to algae blooms and a loss of dissolved oxygen within the lake. Surface water (epilimnion) concentrations of total phosphorus less than 10 ppb are associated with oligotrophic (clean, clear water) conditions. Concentrations greater than 25 ppb are associated with eutrophic (nutrient-rich) conditions.
The total phosphorus in the upper water of Loon Lake ranged from 9 ppb to 10 ppb. The average concentration was 10.0 ppb in the North Basin. The average concentration was also 10.0 ppb in the South Basin. This total phosphorous average value would classify Loon Lake as a late oligotrophic lake.
Chlorophyll-a
Chlorophyll-a is the green pigment in plants used for photosynthesis, and measuring it provides information on the amount of algae (microscopic plants) in lakes. Chlorophyll-a concentrations are also used to classify a lakes trophic status. Concentrations less than 2 ppb are associated with oligotrophic conditions and those greater than 8 ppb are associated with eutrophic conditions.
The chlorophyll-a concentrations in the upper water of Loon Lake ranged from 1.82 ppb to 1.98 ppb. The average concentration was 1.92 ppb in the North Basin. The average concentration was a higher 2.42 ppb in the South Basin. This is indicative of oligotrophic conditions in the North and late oligotrophic to early mesotrophic conditions in the South Basin.
Secchi Disk Transparency
Transparency is a measure of water clarity in lakes and ponds. It is determined by lowering a 20 cm black and white disk (Secchi) into a lake to the depth where it is no longer visible from the surface. This depth is then recorded in meters. Since algae are the main determinant of water clarity in non-stained, low turbidity (suspended silt) lakes, transparency is also used as an indicator of the trophic status of a body of water. Secchi disk transparencies greater than 4.6 meters (15.1 feet) are associated with oligotrophic conditions, while values less than 2 meters (6.6 feet) are associated with eutrophic conditions (DEC & FOLA, 1990).
Secchi disk transparency in Loon Lake was only reported by the AWI staff on their visit on 08/05/08 and it was 5.50 meters in the North basin. Field sheets listing Secchi disk transparency values for 2008 found by volunteers were not returned. Hopefully these sheets can be found and included in the 2009 report. This one value would classify Loon Lake in the North Basin as an oligotrophic lake.
Nitrate
Nitrogen is another essential nutrient for life. Nitrate is an inorganic form of nitrogen that is naturally occurring in the environment. It is also a component of atmospheric pollution. Nitrogen concentrations are usually less than 1 ppm in most lakes. Elevated levels of nitrate concentration may be indicative of lake acidification or wastewater pollution.
The nitrate in the upper water of Loon Lake ranged from 0.00 ppm to 0.10 ppm. The average nitrate concentration was 0.030 ppm in the North Basin. The average nitrate concentration was 0.030 ppm in the South Basin also.
Chloride
Chloride is an anion that occurs naturally in surface waters, though typically in low concentrations. Background concentrations of chloride in Adirondack Lakes are usually less than 1 ppm. Chloride levels 10 ppm and higher is usually indicative of pollution and, if sustained, can alter the distribution and abundance of aquatic plant and animal species. The primary sources of additional chloride in Adirondack lakes are road salt (from winter road de-icing) and wastewater (usually from faulty septic systems). The most salt impacted waters in the Adirondacks usually have chloride concentrations of 100 ppm or less.
The chloride in the upper water of Loon Lake was a very low 4.8 ppm in 2008 in the North Basin. The level in the bottom water was also 4.8 ppm in 2008.
Conductivity
Conductivity is a measure of the ability of water to conduct electric current, and will increase as dissolved minerals build up within a body of water. As a result, conductivity is also an indirect measure of the number of ions in solution, mostly as inorganic substances. High conductivity values (greater than 50 mohms/cm) may be indicative of pollution by road salt runoff or faulty septic systems. Conductivities may be naturally high in water that drains from bogs or marshes. Eutrophic lakes often have conductivities near 100 mohms/cm, but may not be characterized by pollution inputs. Clean, clear-water lakes in our region typically have conductivities up to 30 mohms/cm, but values less than 50 mohms/cm are considered normal.
The conductivity in the upper water of Loon Lake ranged from 41.7 mohms/cm to 43.1 mohms/cm. The average conductivity was 42.4 mohms/cm in the North Basin. . The average conductivity was 39.8 mohms/cm in the South Basin.
Color
The color of water is affected by both dissolved (e.g., metallic ions, organic acids) and suspended (e.g., silt and plant pigments) materials. Water samples are collected and compared to a set of standardized chloroplatinate solutions in order to assess the degree of coloration. The measurement of color is usually used in lake classification to describe the degree to which the water body is stained due to the accumulation of organic acids. The standard for drinking water color, as set by the United States Environmental Protection Agency (US EPA) using the platinum-cobalt method, is 15 Pt-Co. However, dystrophic lakes (heavily stained, often the color of tea) are common in this part of the country, and are usually found in areas with poorly drained soils and large amounts of coniferous vegetation (i.e., pines, spruce, hemlock). Dystrophic lakes usually have color values upwards of 75 Pt-Co.
Color can often be used as a possible index of organic acid content since higher amounts of total organic carbon (TOC) are usually found in colored waters. TOC is important because it can bond with aluminum in water, locking it up within the aquatic system and resulting in possible toxicity to fish (see Aluminum).
The color in the upper water of Loon Lake ranged from 14 Pt-Co to 21 Pt-Co. The average color was 17.7 Pt-Co in the North Basin. The average color was 21.7 Pt-Co in the South Basin.
Aluminum
Aluminum is one of the most abundant elements found within the earth’s crust. Acidic runoff (from rainwater and snowmelt) can leach aluminum out of the soil as it flows into streams and lakes. If a lake is acidic enough, aluminum may also be leached from the sediment at the bottom of it. Low concentrations of aluminum can be toxic to aquatic fauna in acidified water bodies, depending on the type of aluminum available, the amount of dissolved organic carbon available to bond with the aluminum, and the pH of the water. Aluminum can form thick mucus that has been shown to cause gill destruction in aquatic fauna (i.e., fish, insects) and, in cases of prolonged exposure, can cause mortality in native fish populations (Potter, 1982). Aluminum concentrations are reported as mg/L of total dissolved aluminum.
The aluminum in the upper water of Loon Lake was a very low 0.009 ppm and the bottom water was a very low 0.007 ppm in 2008.
Dissolved Oxygen
The dissolved oxygen in a lake is an extremely important parameter to measure. If dissolved oxygen decreases as we approach the bottom of a lake we know that there is a great amount of bacterial decay that is going on. This usually means that there is an abundance of nutrients, like phosphorous that have collected on the lake bottom. Oligotrophic lakes tend to have the same amount of dissolved oxygen from the surface waters to the lake bottom, thus showing very little bacterial decay. Eutrophic lakes tend to have so much decay that their bottom waters will have very little dissolved oxygen. Cold-water fish need 6.0 ppm dissolved oxygen to thrive and reproduce. Warm water fish need 4.0-ppm oxygen.
The dissolved oxygen and temperature profiles for Loon Lake for 2000 - 2004 and 2008 are presented in Appendix A. The dissolved oxygen gradually decreases from the surface to the bottom in Loon Lake. This decrease in dissolved oxygen with depth in Loon Lake is much more pronounced when the readings were taken when the lake was warmer as shown in 2001 and 2008. During both of these years, the water was warmest as shown on the temperature profile and the dissolved oxygen decreased rapidly at a depth between 8 and 10 meters. The oxygen level is sufficient for cold and warm-water fish survival in the surface waters but cold water fish may become stressed in the lower levels of the lake during the warm summer months.
Summary
Loon Lake was a slightly productive late oligotrophic lake during 2008. Based on the results of the 2008 Adirondack Lake Assessment program, the acidity status of Loon Lake is considered to be satisfactory, with a low sensitivity to further acidic inputs.
The trend graphs found in the Appendix only reflect data collected from the North basin or usual sampling location. The South Basin has only been sampled this past season. Eleven years of data are sufficient to detect water quality trends; and it is possible to compare the current data with the data collected in 1998 through 2007. This year, the pH, color and nitrate levels were lower than in 2007. In 2008, the alkalinity, conductivity, total phosphorous, chlorophyll-a, and Secchi disk transparency levels were higher than in 2007. The total phosphorous has been at very low levels the past four years. This low total phosphorous level led to fewer nutrients in the lake available for algal growth. This was reflected by lower chlorophyll-a levels the past few summers and, because there was less algal growth, the secchi disk transparency readings were higher the last five years. Over the entire eleven years of this study, the water quality of Loon Lake has improved greatly. Total phosphorous and chlorophyll-a levels have declined almost every year and this has led to an increase in secchi disk levels since 2002.
The sampling of the lake was switched from five months and one station in the North to three months and two stations one in the North and one in the South. We recommend that this change to two stations continue in 2009. Loon Lake is a long narrow lake with two separate basins and each should be sampled and their water quality compared to see if there are any differences between the two basins.
Literature Cited
DEC & FOLA. (1990). Diet for a Small Lake: A New Yorker’s Guide to Lake Management.
New York State Department of Environmental Conservation & The Federation of Lake
Associations, Inc.: Albany, New York.
Greenberg, A.E., Eaton, A.D., and Leseri, L.A. (editors). (2005). Standard Methods for the
Examination of Water and Wastewater, 21st Edition. American Public Health Association:
Washington, D.C.
Potter, W. (1982). The Effects of Air Pollution and Acid Rain on Fish, Wildlife and Their
Habitats – Lakes. Technical Report FWS/OBS – 80/50.4. United States Fish and Wildlife
Service, Biological Services Program: Washington, D.C.
Appendix A
Water Quality Data